Medicines Australia today welcomed the Prime Minister’s new Ministerial and Cabinet appointments.
“We’re pleased that the Hon Tanya Plibersek MP has retained the Health portfolio,” said Medicines Australia Chief Executive, Dr Brendan Shaw.
“The addition of Medical Research to Ms Plibersek’s responsibilities is also particularly welcome and reflects her ongoing personal interest in this important area.
“On the back of the McKeon Review of Health and Medical Research, the recognition of medical research at the Cabinet level is encouraging.
“We congratulate Senator the Hon Jacinta Collins on her appointment as Minister for Mental Health and Ageing and look forward to working with her in the future.
“We are also encouraged with the appointment of Senator the Hon Kim Carr to the Industry, Innovation, Science and Research portfolio.
“The industry worked well with Senator Carr in his previous stint as Industry Minister on issues like the Pharmaceutical Industry Strategy Group, patents, the Raising the Bar Bill, clinical trials and the R&D tax credit.
“We congratulate him on his appointment and look forward to working with him again on building the future for the Australian medicines industry.
“We also welcome the Hon Richard Marles MP to the Trade portfolio and look forward to working with him on continuing to build the medicines industry’s export and trade performance.
“The Prime Minister’s focus on diversifying the economy by developing manufacturing and innovation in his announcement of his ministerial appointments is to be congratulated.
“The Australian medicines industry represents a real opportunity for the country to build on its competitive advantages in innovation, medical research and exporting to continue to build the industry and support Australia’s post-mining boom future.
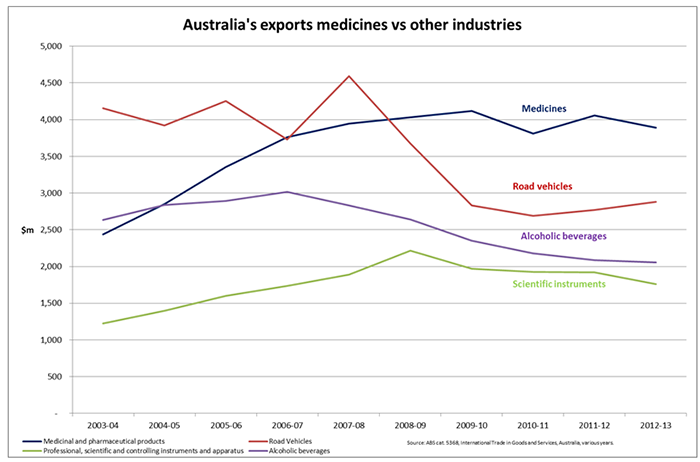
“The Australian medicines industry employs over 13,000 people in high-skilled jobs, exports over $4 billion a year – more than the car or wine industries, invests $1 billion a year in R&D, brings new medicines and vaccines to the community, collaborates extensively with Australia’s public research sector and has a low carbon footprint.
“We have a real opportunity to build on this and grow the industry for the future health of Australians and the Australian economy.”
-ENDS-
Contact Person:
Emma Pearson
Phone: (02) 8281 3280
Email: emma@ogilvyprhealth.com