A Collaborative Assessment of Access to Cancer Medicines in Australia
With approximately 800 cancer medicines currently in industry research pipelines, it is expected over the next decade the number of innovative treatments available in the fight against cancer will increase exponentially.
Australia now finds itself at a pivotal point for both planning and policy development. To help step up the fight against cancer, Medicines Australia’s Oncology Industry Taskforce (OIT) has worked with Deloitte Access Economics to investigate Access to Cancer Medicines in Australia.
This report has found Australia has broadly kept pace with its international peers, who are also grappling with the challenge of how patients can best access specialised, high cost cancer medicines. The report draws together Australian and international research with findings from more than 30 interviews, providing practical and prioritised considerations for Government and policy makers.
The Australian Government is already working hard to ensure these emerging therapies get into the hands of patients. It recently announced plans to streamline the approval process for new medicines. To progress and tackle the emerging challenge of access to cancer medicines, a collaborative approach between doctors, Government, industry and patients is needed.
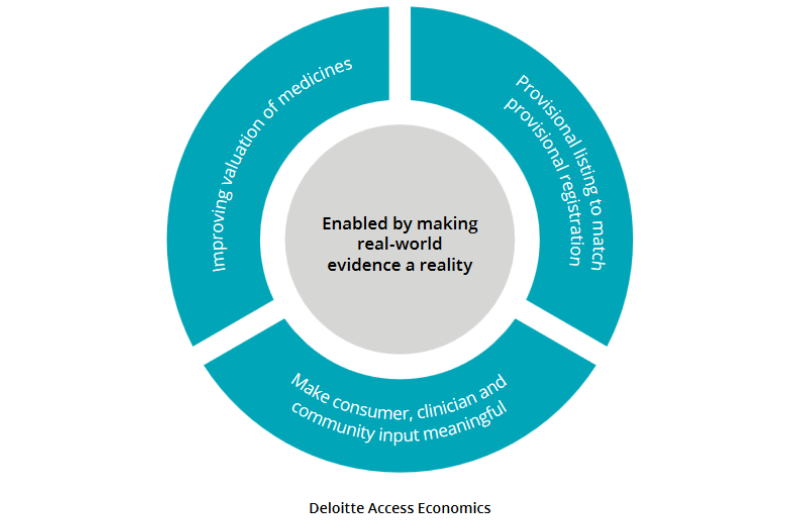
Three key recommendations are:
- A need for greater investment in real-world evidence, to be supported by revised evidence requirements for the valuation of cancer medicines
- The implementation of provisional a drug listing scheme; and
- Further enhancements to consumer, clinician, and community involvement
These recommendations are also captured in the ideas for change diagram above.
In recent years substantial progress towards ensuring improved access to emerging cancer medicines has been made. The OIT remains committed to working collaboratively with government and the community to continue stepping-up the fight against cancer and working to improve patient outcomes.
To find out more, and to read the report in full, click here.