Reforming Australia’s Health Technology Assessment (HTA) System
Medicines Australia is advocating for reform of Australia’s Health Technology Assessment (HTA) system to give Australians faster access to new Pharmaceutical Benefits Scheme (PBS) medicines once they are approved for use by the Therapeutic Goods Administration, now and into the future.
HTA is the process by which medicines are assessed for health benefits and value for money compared to other medicines, before they are recommended for inclusion on the PBS.
The HTA Review Accelerating Access to the Best Medicines for Australians Now and into the Future released in September 2024 found Australia’s system is out-dated and needs urgent reform, with delays to accessing new medicines through the PBS having a profound impact on individual patients. Prior to this Review, Australia’s HTA had not been comprehensively reviewed in more than 30 years.
Implementing the full package of recommended reforms will ensure Australia’s HTA system evolves to keep pace with advancements in medical technologies and delivers faster patient access to new medicines.
HTA Review findings
The Review made 50 recommendations for reform that will:
- 1. Address inequities in access.
- 2. Improve timely access to medicines.
- 3. Improve engagement.
- 4. Invest in HTA capability to make it adaptable and future proof.
Some of the areas for improvement in Australia’s current HTA process identified through the Review include:
- Health technologies are not funded in the shortest time possible.
- Delays are due to the economic evaluation being used as a proxy for negotiation of the prices paid.
- HTA pathways in Australia are more complex than they need to be.
Who will benefit
- Australians will benefit from access to affordable breakthrough, innovative medicines as early as possible.
- The medicines industry will benefit from policy stability and certainty of investment in new medicines, and assessment processes that adapt to rapid advances in medicines, enabling them to funded in Australia as soon as possible.
- The Australian economy will benefit from improved health outcomes, savings generated elsewhere in the health system, and continued investment in research and innovation.
Learn more about some of the key recommendations and what these will mean for therapeutic areas:
Next steps
Medicines Australia is working with the Government, patient advocacy groups, clinicians, health peak bodies and other stakeholders to progress reforms.
An independent Implementation Group will be established in 2024 by the Government to guide implementation of the Review.
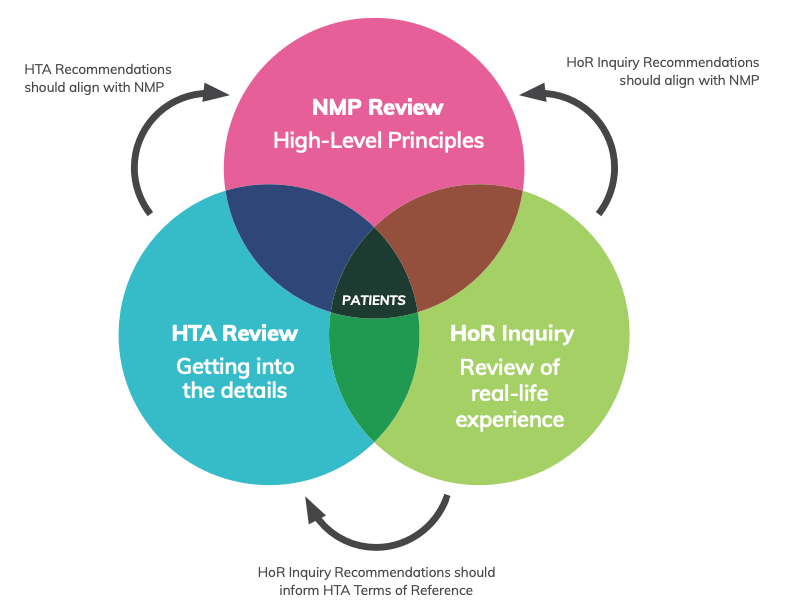
Linking the HTA Reform, NMP Review and HoR Inquiry
The HTA Review follows two other recent reviews and inquiries that were undertaken to ensure Australia’s medicines policy is fit-for-future:
- A 2020 House of Representatives Inquiry which looked at the approval processes for new drugs and novel medical technologies in Australia.
- A review of the National Medicines Policy.
Medicines Australia’s HTA Policy Roundtable
The HTA Policy Roundtable was convened by Medicines Australia in 2024, between the release of the HTA Options Paper and the HTA Review Final Report. Patient, clinician, academic and industry stakeholders participated in the Roundtable, and explored three policy options from the HTA Review Options Paper.
Download the Roundtable report here >>
Medicines Australia’s submissions
Learn more about how Medicines Australia has been involved in the HTA Review:


















