Clinical trial numbers fall for third straight year
New figures from the Therapeutic Goods Administration (see below) showing the third straight fall in the annual number of new clinical trials in Australia highlight the urgent need for Government to deliver on its promise to fix the problem, Medicines Australia chief executive Dr Brendan Shaw said today.
The Government announced on 2 March that it would implement by July 2011 the recommendations of a report prepared by the Clinical Trials Action Group, which was established by the Government to arrest the decline in clinical trials activity.
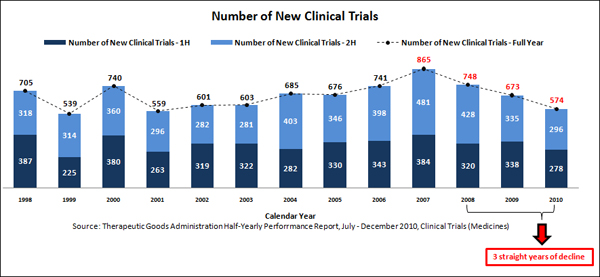
The TGA’s latest Half-Yearly Performance Report shows only 574 clinical trials were started in Australia in 2010. That is the lowest number since 2001 and 99 fewer than 2009.
Dr Shaw said the continued decline in the number of trials in Australia underscored the importance of the Government’s commitment to improve the regulatory framework for clinical trials by July.
“The number of new clinical trials in Australia has fallen by an average of 13 per cent every year since 2007,” Dr Shaw said.
“For the first time ever, we’ve seen three consecutive falls in clinical trial numbers, and a 15 per cent drop in the past year.
“These are not the kinds of records we want to be setting. It’s extremely disappointing.
“The very future of Australia’s $1 billion clinical research industry is at stake. I don’t think that is putting it too strongly.
“Clinical trials deliver significant benefit to the national economy and this is crunch time for the industry.
“The competition for R&D investment from countries in Asia and Europe is extremely fierce. Only with the right policy settings can we hope to grow our R&D industry and keep cutting-edge medical science in Australia.
“That would ensure we keep more of our top scientists in Australia and attract greater investment to our universities and other research institutions.
“Implementing these recommendations will help return Australia to the forefront of medical research and ensure the retention of thousands of high-skill Australian jobs.
“Clinical trials also provide early access to innovative medicines for Australians that otherwise would not be available. Sometimes these are life-saving medicines where there is no other treatment, particularly in cancer therapy and for rare diseases.
“These trials save the Pharmaceutical Benefits Scheme $100 million a year, so the more clinical trials we conduct in Australia the greater the saving for the taxpayer.
“It is very much in the national interest that we seize this opportunity, from both a healthcare and an economic standpoint.”
–ENDS–
Contact Person:
Jamie Nicholson
Media Communications Manager
Phone: 0419 220 293
Email: Jamie.Nicholson@medicinesaustralia.com.au
