New report shows upturn in clinical trials numbers
New figures from the Therapeutic Goods Administration (see below) show that for the first time in four years the number of clinical trials undertaken in Australia has grown, but that activity still lags behind the long-term average.
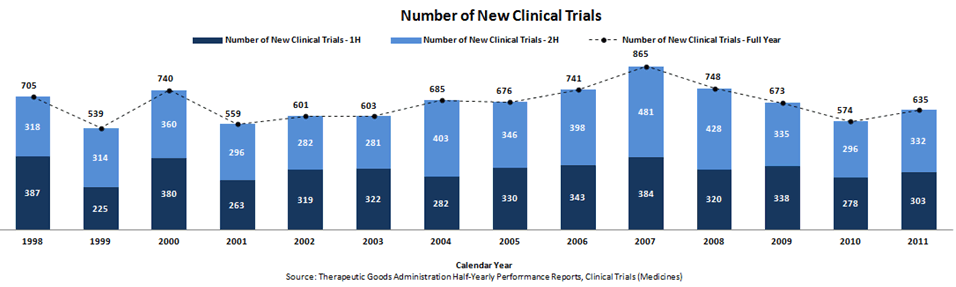
The TGA’s latest Half-Yearly Performance Report shows 635 new clinical trials were begun in Australia in 2011, up from 574 in 2010 but still more than 25 per cent down from the 2007 high of 865 trials.
Medicines Australia chief executive Dr Brendan Shaw said while the upturn was encouraging, there remained serious challenges to Australia’s status as a regional hub for clinical trials.
“The latest figures don’t alleviate the need for urgent policy action to drive microeconomic and regulatory reform to secure the future of clinical trials in Australia,” Dr Shaw said.
“The Government’s commitment last year to a series of regulatory reforms to increase Australia’s competitiveness as a destination for clinical trials may have encouraged some investment over the past year.
“But there has been insufficient progress in implementing these regulatory reforms. In the meantime, we have been losing trials to other countries.
“Australia is recognised globally as having some of the best scientists and research infrastructure in the world and that is an important strategic advantage. However, we are facing fierce international competition for clinical trial investment.
“Australia must act now to ensure we remain competitive.
“There is an urgent need for greater political resolve both at Federal and State level to implement these regulatory reforms expeditiously.
“We are renowned as a country of scientific excellence.
“It is very much in the national interest that we do all we can to keep cutting-edge medical science in Australia.”
The Federal Government committed in 2011 to implement all the recommendations from the Clinical Trials Action Group, which was established by the Government to arrest the decline in clinical trials activity, by July 2011.
However, more than 12 months after the report’s release, the Action Group’s major recommendations that will make a real difference to clinical trial investment have not yet been implemented.
More than 18,000 Australian patients are given early access to innovative medicines, medical devices and diagnostics through clinical trials each year, saving Australian taxpayers $100 million a year.
-ENDS-
Contact Person:
Jamie Nicholson
Media Communications Manager
Phone: 0419 220 293
Email: Jamie.Nicholson@medicinesaustralia.com.au
