New report shows medicines are value for money
Figures in a new publication released today by Medicines Australia show that Australia’s medicines industry makes a major contribution to the health of Australians and the national economy.
The 2nd Edition of Medicines Australia’s Facts Book provides a wealth of information on medicines, the medicines industry and the health of Australians.
Medicines Australia chief executive Dr Brendan Shaw said the report showed that Australians get a good return on investment from the Pharmaceutical Benefits Scheme.
“Our Facts Book shows that for a relatively small investment of public funds by international standards, Australians get good value for money from the PBS,” Dr Shaw said.
“Subsidised medicines in Australia account for just over half of one per cent of GDP or 8 per cent of all health spending.
“For example, that modest investment included Australians receiving 22 million prescriptions for cholesterol lowering medicines to help prevent heart attacks, 20 million prescriptions of medicines to lower blood pressure, and 16 million prescriptions for medicines to treat stomach ulcers.
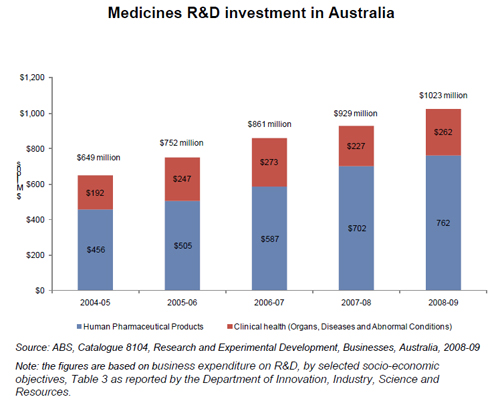
“The Australian medicines industry also produced medicines to treat conditions such as HIV, cancer, arthritis, hepatitis, asthma, diabetes and osteoporosis. These include 12 new medicines added to the PBS during 2009-10.
“Death rates from infectious diseases, respiratory disease, cardiovascular disease and cancer have all fallen over the past two decades.”
Five key facts about Australia’s medicines industry:
- Australia’s medicines industry now exports more and conducts more R&D than the car industry.
- Private industry funds two-thirds of all the clinical trials conducted in Australia – clinical trials are a key part of the innovative process to develop new medicines – with 55 per cent of these conducted through public hospitals.
- The PBS accounts for just 8 per cent of Australia’s total health expenditure.
- In 2010 there were almost 3000 medicines under development worldwide.
- There are over 800 medicines in development to treat cancer.
The Medicines Australia Facts Book is available here
–ENDS–
Contact Person:
Jamie Nicholson
Media Communications Manager
Phone: 0419 220 293
Email: Jamie.Nicholson@medicinesaustralia.com.au