Since 1948 the Pharmaceutical Benefits Scheme (PBS) has provided Australian patients with access to affordable and high-quality, safe and effective medicines when they need them. Underpinned by the National Medicines Policy (NMP) and a strong working relationship between Government and the medicines industry, the PBS has evolved from basic access to antibiotics and painkillers, to a system that has to contend with changing burdens of disease and evolving innovations in treatments.
The PBS has a strong reputation inside and outside of Australia for being one of the best systems available to both patients and taxpayers. It works as part of the national, publicly funded, health care system whereby the government subsidises the cost of approved prescription medicines to make them available and affordable for the Australians who need them.
As at January 2022, patients are required to contribute to this cost via a copayment of $42.501, or $6.80 for concession card holders. The government pays for the cost of the medicines, as well as for the distribution and associated dispensing activities.
The PBS relies on a complex supply chain;
- The medicines manufacturer who invents, discovers, develops, manufactures and supplies a medicine
- The medical and health professionals who diagnose, select and prescribe a medicine
- The network of wholesale distributors
- The community, hospital and pharmacy outlets who dispense the medicine to the patient.
Under the National Health Act 1953, for a medicine to be added to the PBS, it must be recommended by the independent Pharmaceutical Benefits Advisory Committee (PBAC). In fact, the Minister for Health (or their Delegate) cannot list a medicine on the PBS without a positive recommendation from the PBAC.
A submission to consider a new medicine on the PBS can be made by industry sponsors of medicines and medicinal products, medical bodies, health professionals, or private individuals and their representatives. However, they are most commonly made by the pharmaceutical sponsor or manufacturer, as they have the scope of evidence required to support an application.
1. The Medicine Approval Process
To achieve medicines approval in Australia, the sponsor must first apply to the Therapeutic Goods Administration (TGA). The TGA is responsible for assessing the package of evidence collected after several years of research, to verify the claimed quality, safety and efficacy of the medicine. This process takes about one year and includes specialist evaluation and analysis of all data collected during the several years of investigation of the new treatment.
Once the TGA approves a medicine, it is listed on the Australian Register for Therapeutic Goods (ARTG). At this point, the medicine is considered to have “TGA registration” or “Marketing Authorisation” and can legally be sold in Australia. The medicine is then subject to ongoing, continuous monitoring, known as pharmacovigilance, by the TGA and the manufacturer to ensure the continued quality, safety and efficacy of the medicine.
The registration approval process also includes the review and approval by the TGA of Product Information (PI) and Consumer Medicines Information (CMI), as key documents to ensure the quality, safe and wise selection and appropriate use of medicines by the prescriber and the consumer2.
2. Medicines Listing on the PBS
To list on the PBS, the manufacturer of the medicine needs to submit a package of clinical and economic evidence to the PBAC, to demonstrate the medicines relative safety and efficacy and cost effectiveness compared to alternative treatments. The PBAC consists of doctors, specialists, health professionals, health economists and consumer representatives, and is responsible for recommending to the Minister for Health which medicines are value for money and should be subsidised through the PBS at a cost-effective price.
Note – Applications for PBS listing can be made in parallel to the application for ARTG registration, aimed at speeding up the time for medicine approval and reimbursement.
Once a medicine receives a positive recommendation from the PBAC, the Department of Health commences negotiations with the medicine manufacturer regarding public price and volume. This is formalised in a contract between the Commonwealth Government and the manufacture3.
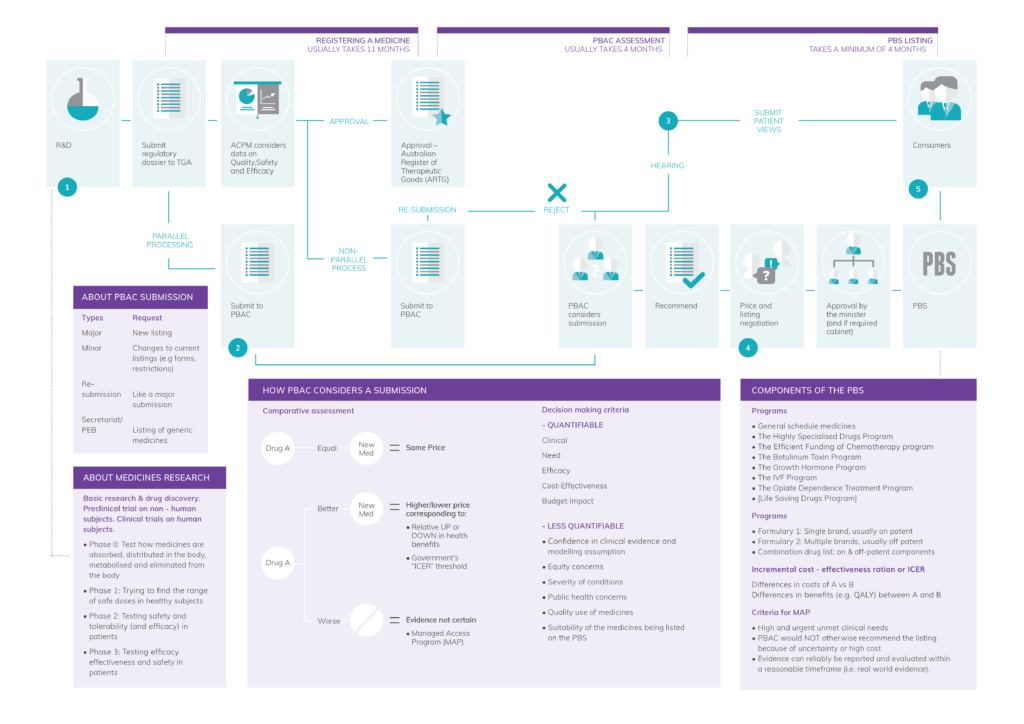
Once the medicine is listed on the PBS there are further rules governing how the reimbursed, cost effective price can change, with statutory price reductions mandated at specific intervals and other reference comparisons made to the prices of similar listed medicines to reduce prices over time.
More than two-thirds of Australians agree that the PBS and the innovative medicines industry delivers a more prosperous future for Australia.4 Never before has this been at the forefront of the community’s mind, than during the COVID-19 pandemic and the understanding of the critical role played by the medicines industry.
- It is expected that the General Patient copay will be reduced by $12.50 later in 2022.
- The Pharmaceutical Benefits Scheme in Australia; An Explainer on System Components, GSK. Available at: https://au.gsk.com/en-au/responsibility/our-policy-advocacy/pulling-back-the-curtain/
- Positive PBAC recommendations need approval from the Minister (or their Delegate) to be listed, and medicines costing over $20m in any year of the forward estimates will also require Cabinet approval.
- Nielsen Research (2019), “Medicines Australia Consumer Sentiment Index”.
