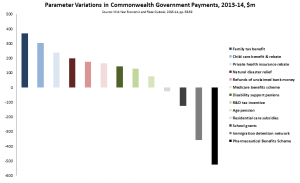
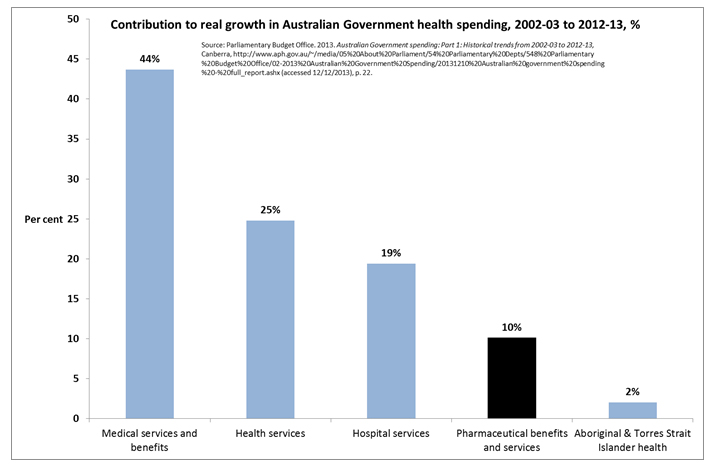
PBS expenditure is contained and sustainable
Government expenditure on the Pharmaceutical Benefits Scheme (PBS) is currently not only sustainable, but actually declined last financial year.
In the past few months, major reports from three separate government agencies have confirmed that PBS expenditure is contained and that price disclosure is delivering savings far in excess of what was expected.
The regular Medicines Partnership of Australia (MPA) PBS Scorecard update released today outlines the current state and trajectory of PBS spending, as confirmed by the Department of Health, the Federal Treasury, and the Productivity Commission.
The Medicines Partnership welcomes the statement from the Minister for Health, Peter Dutton, that Australia needs “to have a national discussion about who pays for what and how the Government pays going forward.”
As part of that national discussion it is important to acknowledge that price disclosure has been and continues to be an extremely effective tool in ensuring that PBS expenditure remains contained and sustainable.
The most current update on PBS expenditure is in the Medicines Partnership of Australia scorecard, issued today.
The Medicines Partnership of Australia is: The Pharmacy Guild, Medicines Australia, the Generic Medicines Industry Association, the Australian Self-Medication Industry, the Pharmaceutical Society of Australia and the National Pharmaceutical Services Association.
Click here to view the PBS Scorecard (please follow link)
-ENDS-
Contact Person:
Alexia Vlahos
Phone: (02) 6122 8503
Email: Alexia.Vlahos@medicinesaustralia.com.au